Function Annotation for BL2970549
spin
Assembly
Download
| Ligand ID | NUC pairing detail | Ligand Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|---|
| DMS | N/A | Dimethyl sulfoxide | "C2 H6 O S" | 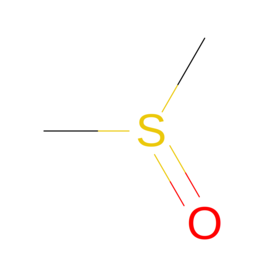 |
Reference
| Ligand ID | NUC pairing detail | Ligand Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|---|
| DMS | N/A | Dimethyl sulfoxide | "C2 H6 O S" |  |