Function Annotation for BL2908925
spin
Assembly
Download
| Ligand ID | NUC pairing detail | Ligand Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|---|
| MG | N/A | Magnesium(2+) | Mg | 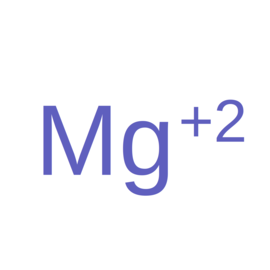 |
Reference
| Ligand ID | NUC pairing detail | Ligand Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|---|
| MG | N/A | Magnesium(2+) | Mg | 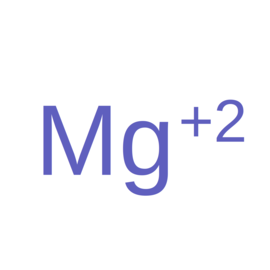 |