Function Annotation for BL8409
Spin
Receptor
Ligand
Download
| Ligand ID | Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|
| IOD | Iodide | I | 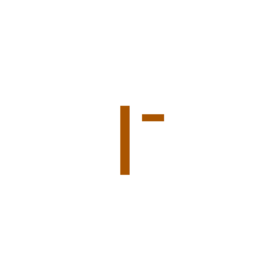 |
Binding affinity
| Experimental affinity | N/A | |||
| Predicted affinity | N/A, |
|||
Surface area (A2)
| Surface area of receptor | 12747.64 |
| Surface area of ligand | 143.56 |
| Surface area of complex | 12808.68 |
| Interface area | 41.26 |
Reference