Function Annotation for BL706
The structure is too big.
Click here to show 3D view, it may take a long time or fail,
depending on the status of the server.
Receptor
Assembly ID: 1al2_6
Ligand
Download
| Ligand ID | Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|
| MYR | Myristic acid | "C14 H28 O2" | 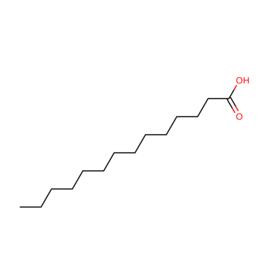 |
Binding affinity
| Experimental affinity | N/A | |||
| Predicted affinity | N/A, |
|||
Surface area
| Surface area of receptor(A2) | N/A |
| Surface area of ligand(A2) | N/A |
| Surface area of complex(A2) | N/A |
| Interface area(A2) | N/A |
Reference