Function Annotation for BL6672
| Ligand ID | Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|
| MG | Magnesium(2+) | Mg | 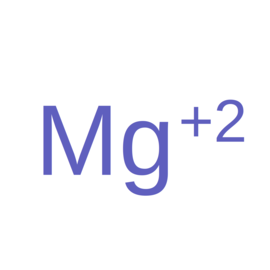 |
Binding affinity
| Experimental affinity | N/A | |||
| Predicted affinity | N/A, |
|||
Surface area (A2)
| Surface area of receptor | 17475.14 |
| Surface area of ligand | 123.9 |
| Surface area of complex | 17437.57 |
| Interface area | 80.74 |
Reference