Function Annotation for BL3256
Spin
Receptor
Ligand
Download
| Ligand ID | Name | Synonyms | Formula | 2D structure |
|---|---|---|---|---|
| CMO | Carbon monoxide | "C O" | 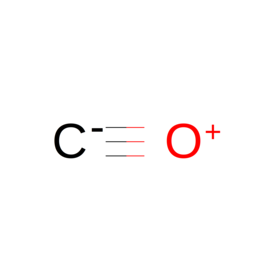 |
Binding affinity
| Experimental affinity | N/A | |||
| Predicted affinity | N/A, |
|||
Surface area (A2)
| Surface area of receptor | 24669.71 |
| Surface area of ligand | 136.85 |
| Surface area of complex | 24630.75 |
| Interface area | 87.9 |
Reference